Long-term memory is an essential process for human life
Without long-term memory, we would not be able to remember our past, interpret our present, or predict our future. We would have little personal identity and functioning in a world that continues to grow in complexity would be near impossible. Our research goal in the Wood lab is to understand the molecular mechanisms underlying normal long-term memory processes so that we may be able to one day develop approaches to ameliorate memory dysfunction associated with intellectual disability disorders as well as age-dependent memory impairment. In addition, because memory circuits in the brain are affected by drugs of abuse, we also examine memory processes associated with drugs of abuse, and how to extinguish those memories. Please read below for brief summaries of current projects in the lab.
It has been known for over 50 years that gene expression is required for long-term memory formation. Yet exactly how gene expression is regulated in neurons engaged in memory formation remains an area of active investigation. One of the most exciting discoveries recently made is that the epigenome (made of your genomic DNA and the proteins that package that DNA into the nucleus of a cell) is essential for dynamic, and also incredibly stable, regulation of gene expression in neurons. Thus, the enzymes that regulate the epigenome are pivotal for the control of gene expression required for long-term changes in neuronal function underlying memory processes.
Our lab focuses on two main enzyme complexes that regulate the epigenome: histone modification and nucleosome remodeling. With regard to histone modification, we work on histone acetyltransferases (HATs), which facilitate gene expression and positively regulate memory formation. Impaired HAT function can lead to severe intellectual disability and cognitive dysfunction in humans (e.g. Rubinstein-Taybi Syndrome). On the other hand, histone deacetylases (HDACs) repress gene expression and negatively regulate memory formation. HDACs can be thought of a molecular brake pad that may prevent you from encoding everything that you experience. We have found that inhibition of that brake pad with pharmacological inhibitors (HDAC inhibitors) can transform a learning event that would not normally be encoded into long-term memory into an event that is now encoded robustly into long-term memory, and can also generate a form of memory that persists beyond the point at which normal long-term memory would fail. These kinds of discoveries demonstrate the power that epigenetic mechanisms have in controlling neuronal function and ultimately memory processes.
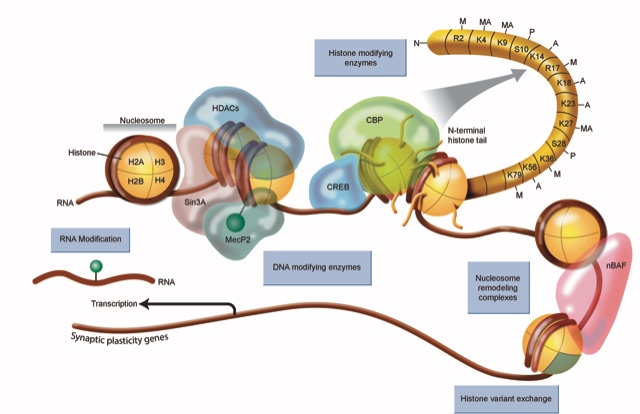
With regard to nucleosome remodeling, we work on the neuronal BAF complex, which is a large protein complex that dynamically regulates chromatin structure to control gene expression. Mutations in the genes that code for proteins in the BAF complex are thought to cause several different kinds of intellectual disability disorders, including Autism Spectrum Disorder. Our lab works on several different subunits of the BAF complex to understand how this complex regulates gene expression required for synaptic plasticity (a form of communication between neurons) and memory formation.
We also study the role of these epigenetic mechanisms in the normal aging brain, as well as the aging brain in the context of neurodegenerative disease. Across species, a hallmark of aging is the repression of gene expression required for memory formation. We examine how that occurs, as well as how to reverse that process. For example, HDAC inhibition in the aging brain significantly enhances synaptic plasticity and memory formation. Part of our work in this area involves exercise. We have a collaboration with Dr. Carl Cotman’s lab here at UC Irvine that examines how the epigenome encodes a molecular memory of past exercise history that subsequently affects memory formation. Again, one of the many exciting and unusual properties that the epigenome generates.
With regard to addiction, we study how drugs of abuse engage enzymes that regulate the epigenome, such as histone modifying enzymes and nucleosome remodeling enzymes described above, which we believe are part of the mechanism of how drugs give rise to incredibly persistent changes in neuronal function that characterize the long-lasting effect of drugs on the rewards circuits of the brain. Furthermore, because the reward and memory circuits of the brain are interrelated and engage each other, we also examine how manipulating the epigenome may hold a key to unlock the ability to extinguish drug-associated memories in a persistent manner that may possibly prevent relapse like behaviors. Current projects in the lab center around how epigenetic mechanisms are engaged by drugs of abuse and how epigenetic mechanisms are involved in cell-type specific and circuit-specific mechanisms of various drug-related processes in the nucleus accumbens, ventral tegmental area, and medial habenula.
Of course, we couldn’t do any of this with support from the National Institute on Drug Abuse, the National Institute on Aging, the National Institute of Mental Health, private foundations including the Hewitt Foundation, and collaborations with industry partners.
